
Medical Devices Registration Process in South Africa by SAHPRA
Country Profile Geographical Location:South Africa is located at the southern tip of the African continent, bordered by Namibia, Botswana, Zimbabwe, ...
Medical Devices and IVDs Regulation and Registration Process in Ghana by Ghana Food and Drug Authority (Ghana-FDA)
The regulation and registration of medical devices and in vitro diagnostic (IVD) devices in Ghana are overseen by the Ghana ...
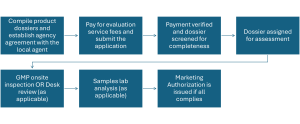
Medicines Regulatory System and Registration Process in Ethiopia by Ethiopian Food and Drug Authority (EFDA)
Ensuring the safety, efficacy, and quality of medicines is paramount for public health. In Ethiopia, this responsibility falls under the ...

Medical device registration process in Kenya by Pharmacy and Poisons Board
Kenya is one of African major countries who have established a regulatory framework for medical devices. The country performs all ...
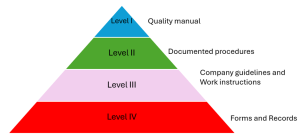
Medical device Quality management system (ISO 13485) mandatory documents templates
The ISO 13485- Medical devices- Quality management system- for regulatory purposes is an international standard developed by a recognized international ...

Medical Device Market Opportunities in Ethiopia
The medical devices market in Ethiopia is on the rise, driven by different factors. A growing population, increasing prevalence of ...

Medicines and medical devices special import permit by Ethiopian Food and Drug Authority
This blog simplifies the procedures and guidelines set for medicines and medical devices special import permit by Ethiopian Food and ...

Regulation of Borderline medical devices by Ethiopian Food and Drug Authority (EFDA)
The world of medical products can be complex, especially when it comes to borderline products that blur the lines between ...

Simplifying Medical Device Approval in Ethiopia: A Guide to Low-Risk Medical Devices Registration by EFDA
The Ethiopian market is seeing a rise in medical devices, and ensuring their safety and effectiveness is crucial. The Food ...
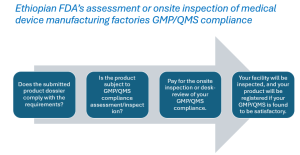
Medical device manufacturing sites’ Quality management system audit by Ethiopian Food and Drug Authority
Ethiopian Food and Drug Authority is a governmental organization mandated by Food and Medicine Administration Proclamation 1112/2019, that has been ...
