Ensuring the safety, efficacy, and quality of medicines is paramount for public health. In Ethiopia, this responsibility falls under the purview of the Ethiopian Food and Drug Authority (EFDA), which has established comprehensive guidelines to perform the regulation duties of the medicinal products. This blog post provides an overview of the current Medicines Regulatory System and Registration Process in Ethiopia by EFDA.
Understanding EFDA’s Role
The EFDA is the national regulatory body responsible for safeguarding public health by ensuring that food, medicines, medical devices and other health-related products meet established standards and other relevant regulatory requirements. It achieves this through a robust regulatory framework that encompasses the evaluation, approval, and monitoring of these regulated products.
Key Regulatory Documents
Several documents including proclamation, regulation, directives and guidelines form the backbone of Ethiopia’s medicines regulatory system. Notably:
Food and Medicine Administration Proclamation- 1112/2019– this is a legal document that empowered EFDA to perform all regulatory activities to ensure the safety, effectiveness and overall acceptability of all regulated products. Unlike its previous version, this proclamation significantly broadened and clarified EFDA’s responsibilities, including its role in the regulation of medicines, medical devices and others.
Guideline for Registration of Medicines (2024): This document serves as technical guidance for both assessors and applicants, detailing the documentation required for the registration of medicines. It provides recommendations on the quality, safety, and efficacy information for both Active Pharmaceutical Ingredients (API) and Finished Pharmaceutical Products (FPP) that should be submitted to the Authority.
Guideline for Conditional Approval of Medicines: This guideline facilitates conditional approval, permitting applicants to obtain a time-limited provisional registration of medicines.
Guideline for Renewal of Medicines Marketing Authorization: This document outlines the procedures for renewing the marketing authorization of medicines, ensuring continued compliance with regulatory standards.
The list is not exhaustive. The EFDA has all legally binding and non-binding regulatory documents necessary to carry out the premarket, placing-on-market and post-market regulation of medicinal products. Ethiobiomedical recommends manufacturers and applicants to access those documents and fully understand before submitting their applications. Please use the Authority’s official website to access the documents here- All Published documents of EFDA.
The Registration Process of Medicines
The EFDA’s registration process is designed to be thorough and transparent, ensuring that only safe, efficacious, and quality-assured medicines are available in the Ethiopian market.
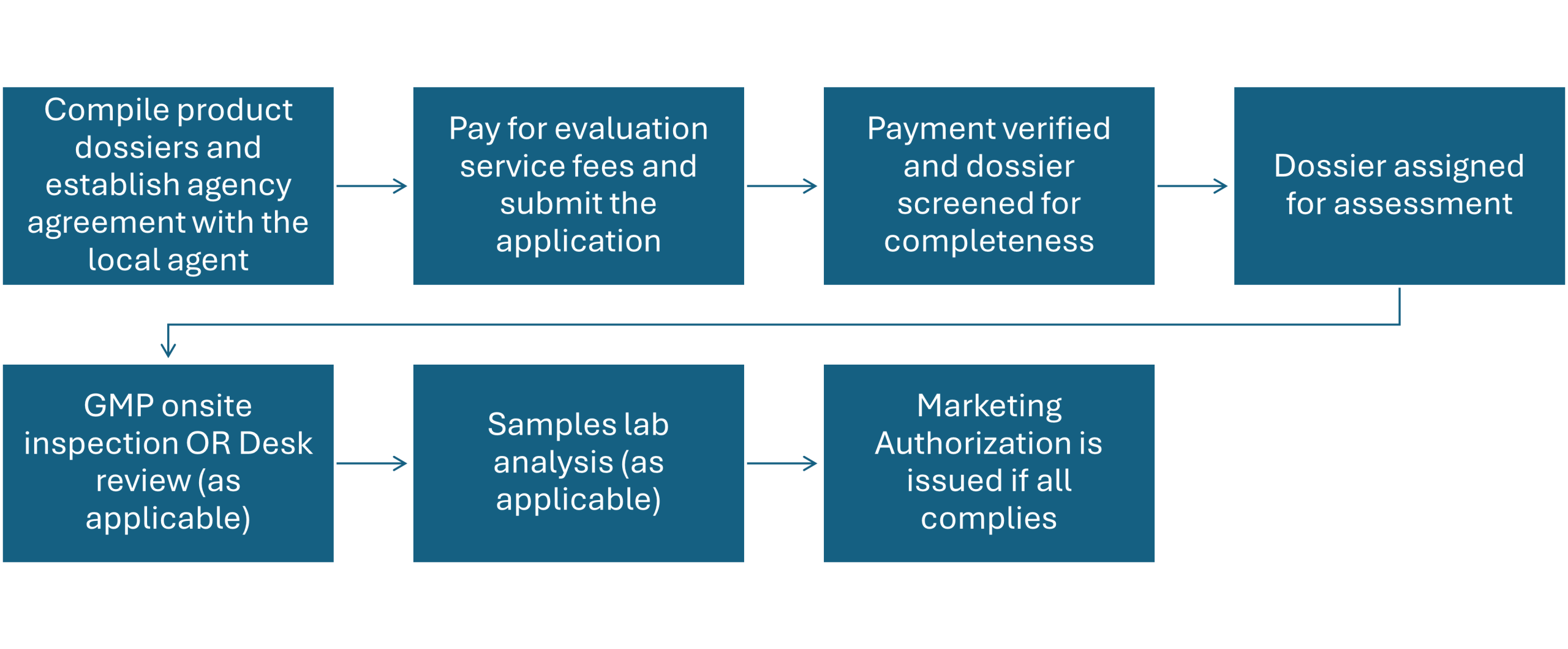
Pre-Submission Preparation– First, you, as a product owner or applicant, should consider compiling comprehensive dossiers by preparing appropriate documents including administrative documents, quality data, non-clinical and clinical information, and labeling details. Next, there should be an agreement between the manufacturer and a local agent responsible for importation and distribution that is established through agency agreement (please check the format in the relevant EFDA’s document).
Application Submission: Applicants must submit a comprehensive dossier through the EFDA’s Electronic Regulatory Information System (eRIS). The dossier should include administrative documents, quality data, non-clinical and clinical information, and product information.
Dossier Assessment: The EFDA conducts a rigorous evaluation of the submitted dossier, focusing on the medicine’s quality, safety, and efficacy. This assessment ensures that the product meets national and international standards as well as relevant regulatory requirements.
Current Good Manufacturing Practice (GMP) Inspection: The manufacturing facility is inspected to verify compliance with GMP requirements, ensuring that the medicine is produced in a controlled and quality-assured environment consistently.
Laboratory Testing: Samples of the medicine may be subjected to laboratory analysis to confirm their quality attributes.
Approval and Registration: Upon satisfactory completion of the assessment, inspections, and lab analysis, the EFDA grants marketing authorization, allowing the medicine to be marketed and distributed in Ethiopia. The marketing authorizations are valid for five years and are subject to renewal, and any changes to the product must be reported and approved by the EFDA to maintain compliance.
Post-Marketing Surveillance: After approval, the EFDA continues to monitor the medicine’s safety and efficacy through pharmacovigilance activities.
Special Considerations
Low-Risk Medicines: The EFDA has established specific guidelines for the registration of low-risk medicines, streamlining the process for products that meet certain criteria.
Conditional Approvals: In certain circumstances, the EFDA may grant conditional approvals to expedite access to essential medicines, subject to specific obligations and timeframes.
Reliance approach- Medicinal products that have been approved by reference regulatory agencies and trusted institutions (e.g., WHO) recognized by the Authority may undergo abbreviated assessment and offsite GMP desk review.
Application Types and Timeframes
The EFDA recognizes various application types and approval pathways, each with specific requirements and processing times:
| Application Type and approval pathway | Description | Estimated Timeframe |
| New regular application | Full dossier submission for new medicines. | 10 months |
| Abbreviated New Application | Reliance based- for medicines approved by SRAs including WHO. | 4 months |
| Low-risk medicines application | For assessment of low-risk medicines with minimal requirements | 3 months |
| Fast-track approval pathway | Medicines for priority diseases (eg. HIV, malaria, TB, etc.) and locally manufactured medicines | 2 months |
| Re-registration | Renewal of marketing authorization for existing products. | 3 months |
| Major Variation | Significant changes affecting product quality, safety, or efficacy. | 3 months |
| Minor Variation | Minor changes not affecting product quality, safety, or efficacy. | 2 months |
| Conditional Approval | Provisional registration under specific conditions. | 3 months |
Ethiobiomedical systematically works to ensure companies who are interested in registering and marketing their medicines and medical devices utilize the appropriate and seamless path to meet their dream. Ethiobiomedical does this by professional handling of marketing authorization and post-registration mandatory activities of the product owners required by the Authority. Please contact us for getting consultancy supports to register your products in Ethiopia.

Biomedical Teconolgy